A new TB treatment called sorfequiline has shown stronger action against the bacteria than existing drugs in early-stage trial results.
The antibiotic demonstrated what researchers describe as enhanced effectiveness against TB bacteria with a comparable safety profile to current treatments.
The findings could lead to shorter treatment times and improved cure rates for a disease that killed 1.23 million people globally last year.
Dr William Brumskine is clinical research site leader at the Aurum Institute in Rustenburg, South Africa.
He said: “The hope of having a universal regimen that is shorter, that has less side-effects, is you will have less individuals coming in for clinic visits [and so] the health care providers will have more time to give individual care to patients.”
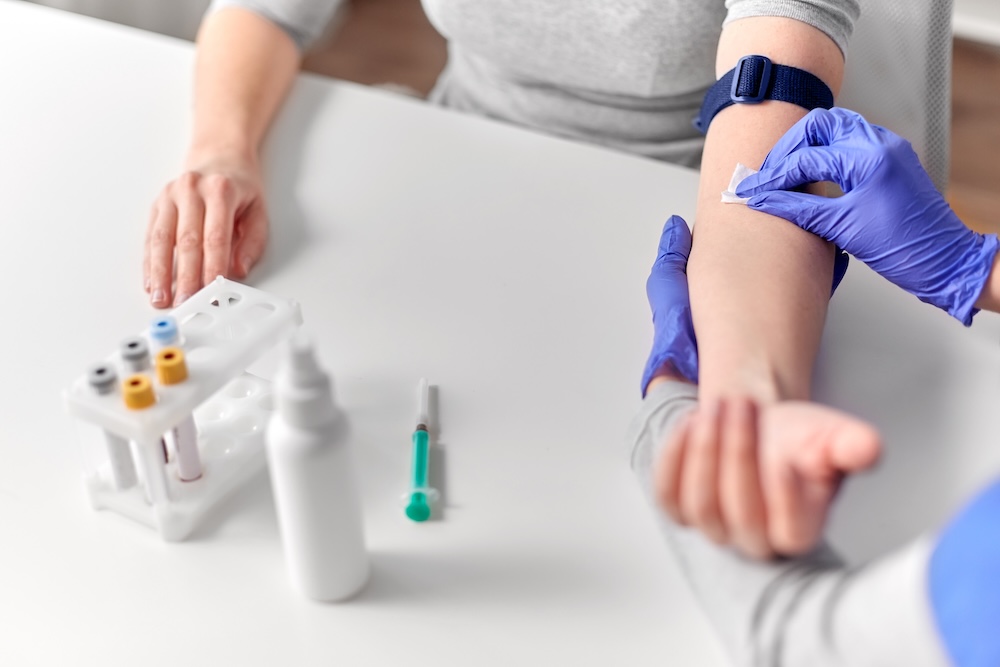
The trial involved 309 patients across 22 sites in South Africa, the Philippines, Georgia, Tanzania and Uganda, testing different dose regimens.
All participants had drug-sensitive tuberculosis, which responds to standard drug combinations, though researchers believe the treatment could also help patients with drug-resistant strains.
Dr Maria Beumont of the TB Alliance said a sorfequiline-based regimen could potentially be used for any patient testing positive for TB, without waiting for laboratory results to identify the specific strain.
The World Health Organization reported last week that 10.7 million people fell ill with TB in 2023, making it the leading infectious cause of death globally.
Progress against the disease is falling short of UN targets to end TB as a public health threat by 2030.
Current treatment for drug-resistant TB, introduced in 2019, successfully treats 90 per cent of patients within six months.
A decade ago, patients faced treatment lasting 18 months or more with only a 50 per cent cure rate.
Dr Maria Beumont, vice-president of TB Alliance, said: “I can just put you on a treatment while I’m waiting to understand exactly what your situation is.
“I don’t need to wait to get information back and classify you as drug sensitive, this or that regimen. There is no need to go through all of that.”
Beaumont described positive early feedback from trial sites: “It’s incredible when you start getting these little anecdotes from the sites [such as]: ‘This patient got cured so fast. I don’t know what arm [of the trial] he was on, but wow, I’ve never seen this before.'”
TB Alliance plans to launch a phase-3 clinical trial in 2026.