A British medical technology company is developing the first and only professional medical rule-out screening test for skin lesions suspected of melanoma.
Moletest (Scotland) Ltd says its novel screening test could reduce the number of unnecessary dermatology referrals to secondary care by over 50%, with the potential to save the NHS £125M per annum.
Since doctors lack a completely accurate way of screening out melanoma, the nomela® test has been developed to assist the process, fulfilling an unmet need and reducing the burden on the healthcare system.
The nomela® uses signal processing and a series of five algorithms to differentiate lesions [a part of the skin with an abnormal growth or appearance].
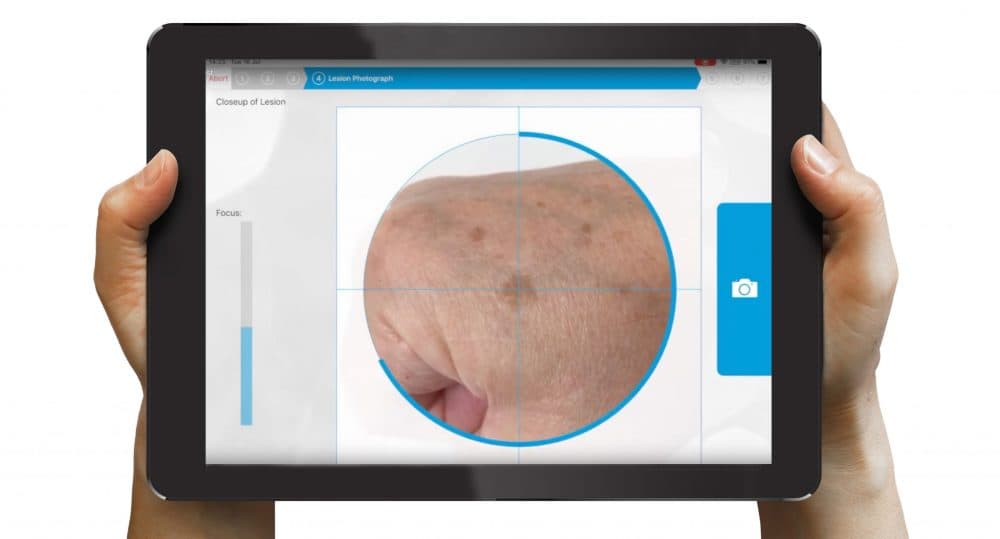
GPs and other primary care professionals use a dedicated nomela® iPad to take high-quality images of the suspect skin lesion, which are analysed to provide an instant result, either “no evidence of melanoma” or “melanoma not excluded”.
Bruce Murray, technical director at Moletest says: “There’s nobody else attempting to achieve this differentiation of lesions that are not melanoma using signal processing. Most of them appear to be only using a single algorithm, which we think isn’t good enough.
“nomela® acts as an aid to help medical professionals manage their patients better. We’re turning the iPad Pro into a bespoke medical device. In this way, we can achieve a huge degree of security, as it won’t be running a browser or email or any other applications; it will only be running our software.
“The devices will be under our central control so we have the ability to turn it off remotely if needed and it will only communicate with end-to-end encryption to our secure server.”
Most skin lesions are harmless but some may be a melanoma, the most aggressive form of skin cancer. Medical advice is that anyone who is concerned about a mole, especially if it is new or changing in size, shape, or colour, should visit a GP to have it examined.
If the GP is uncertain or suspects melanoma, then urgent referral to a dermatologist within 14 days for further assessment is mandatory, though some 97% of such moles are found to be benign. If the dermatologist or plastic surgeon remains concerned, then a biopsy (removal of some or all of the affected skin) is recommended.
The first clinical trial of the nomela® were carried out in conjunction with NHS Lanarkshire in 2016, results from which are due to be published by the British Association of Dermatologists Annual Meeting in September 2020.
These show that, with ranges set by nomela® at 100% sensitivity for melanoma, 53% of non-melanoma lesions may be assessed as benign. By extrapolation, if used by a GP, nomela® could reduce the number of unnecessary dermatology referrals by more than 50%,
Murray says: “When we ran our first clinical trials up in Scotland, we found that for every hundred cases being referred through to a specialist, there were actually only three melanomas.
“So, what you’ve got is a huge overflow of people joining acute care specialists. And it’s worse than that, because if you’re told that a lesion on your body may be cancer, you’re going to be worried to death. When in fact, the likelihood of you having cancer is only 3%.”
The Scottish company has now restarted its clinical trial with Addenbrooke’s Hospital (Cambridge) which, along with almost all other UK trials, was suspended by the National Institute for Health Research in March due to the COVID-19 crisis. Moletest says the trial aims to illustrate the test’s capacity to improve the patient pathway in dermatology care and its potential for cost savings.
Murray says: “If you go to the dermatology ward at Addenbrooke’s Hospital and doctors are worried you have melanoma, you will be sent for a biopsy, which may involve cutting the lesion out and putting it under a microscope for diagnosis.
“We’re using nomela® just before they remove the lesion. So, if we can get our results backed up with the biopsy result, we’ve got a very strong case that the system works.”
An obvious risk to the test is the possibility of it failing to identify a cancerous lesion. An issue that Moletest says it will address by providing training and keeping the final decision in the hands of the medical professional.
“There is absolutely nothing out there that never gets it wrong,” Murray says. “For example, the sensitivity of mammograms, that are so relied on, are appallingly low.
“A GP may only see a real melanoma once every three years, so they don’t see them often enough to be sure. This means they tend to err on the side of caution. What nomela® does is give them a kind of snapshot reassurance.”
Moletest is having initial conversations with the Federal Drug Administration about starting clinical trials in the US and is currently seeking to develop collaborations with primary care clinicians in the UK, such as GP practices, hospital groups and pharmacists. The company is also seeking ways to accelerate the realisation of the nomela® device’s commercial potential, both in the UK and globally.
Murray says: “Everything we’ve done so far has been within a hospital setting. Primary care is more of a difficult area to run clinical trials, so what we’re looking to carry out is a sort of beta test.
“This means it wouldn’t affect the patient pathway, but we will get feedback from GPs and practice nurses on using nomela® on patients that agree to it. We want to see whether all the work we’ve done to simplify the user interface has been effective and to make sure everything works in primary care.”
“nomela® can deliver true innovation within this market, demonstrating the three dimensions of value that are critical in healthcare, including clinical benefit, patient quality of life and economic value.”





